- July 26, 2024
-
-
Loading

Loading

Since March 2020, COVID-19’s effects have been devastating. As of Dec. 21, 2020, more than 1.2 million Floridians had been infected — more than 68,000 in Orange County alone — and more than 20,000 have died in the state. Around the country, the death toll has risen to more than 318,000.
Along with the death and sickness brought on by the virus, the area — and beyond — has seen rises in unemployment and the decimation of the economy. That’s why many have been waiting for some kind of relief, especially in the form of a vaccine.
That relief is now here in the form of two vaccines — the first from Pfizer/BioNTec and the second from Moderna. The quickness of the whole process — known as Operation Warp Speed — has been watched closely by many medical organizations, including Community Health Centers.
“(Although) the timeline may seem long to us in the middle of a pandemic, the timeline for this vaccine — development, approval and all the studies that went into it — is, to put how the government defined this, it’s warp speed,” CHC CEO and President Debra Andree said. “In the grand scope of all things being relative, it was remarkable how quickly they developed it, studied it and got it ready for distribution and the approval was done.”
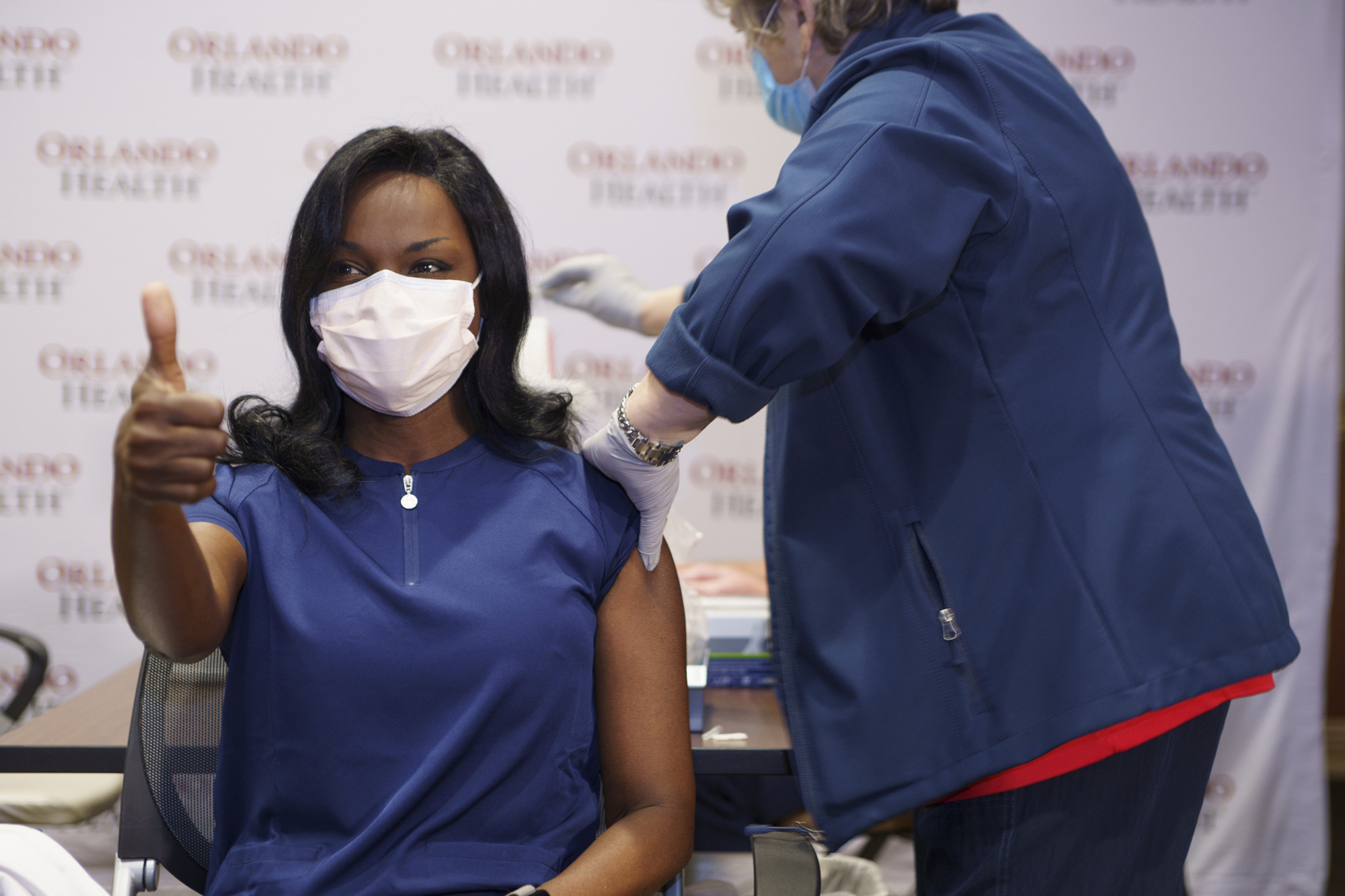
On Dec. 11, 2020, the U.S. Food and Drug Administration issued the first emergency-use approval of the Pfizer/BioNTec vaccine.
The vaccine itself requires two different shots — 21 days apart — and based on clinical trials, the vaccine has shown to be 95% effective in helping to prevent COVID-19. The Moderna vaccine goes by the same two-shot model but is given 28 days apart.
The vaccine is being administered first to those medical professionals on the front lines of dealing with the pandemic.
AdventHealth, tapped as one of the first sites to store and distribute the vaccine by the state, received an initial 20,000 inoculations it used on front line team members. It also shared the vaccine with other local medical facilities, including Nemours Children’s Health System and Orlando Health.
“We are convinced that this is a safe and effective vaccine,” said Dr. Neil Finkler, chief medical officer at AdventHealth hospitals in Orange, Osceola and Seminole counties. “This is a watershed moment.”
Dr. Steven Smith, AdventHealth’s chief scientific officer, said AdventHealth had been in preparation for the last three months for this moment, and that there were two distribution centers for employees at the facility in Celebration and at the main campus in downtown Orlando.
A week after AdventHealth received the Pfizer/BioNTec vaccine, the Florida Department of Health in Orange County received its first shipment of 16,000 doses of the Moderna vaccine Tuesday, Dec. 22, which it administered to Orange County EMTs and paramedics who are included in the state’s priority 1A distribution list.
“In the grand scope of all things being relative, it was remarkable how quickly they developed it, studied it and got it ready for distribution and the approval was done.”
— Debra Andree, Comunity Health Centers CEO and President
One of the big differentiating factors between the two vaccines is the temperature at which they must be stored. The Pfizer/BioNTec vaccine has to kept stored below freezing at minus-70 degrees Celsius — for reference, winters in Antartica are warmer — while the Moderna vaccine only requires storage at minus-20 degrees Celsius, which is closer to the temperature in a standard freezer.
Hospitals have this kind of cold-storage capacity, but some medical facilities — such as PremierMed Family Practice and Sports Medicine — are more limited on space to be able to distribute the vaccine.
Andree said the Florida Department of Health told her CHC could expect its first shipments as early as the first two weeks of January. It has been a long time of preparation to receive the vaccine.
“We based our storage order on what we thought would become available for us, and then we have a back-up plan with Orange County in the event our storage was not here in time or insufficient,” Andree said. “We didn’t want to be left with no storage capacity, so we thought it prudent to order ahead of time, and that has played out to our advantage.”
Much like how the vaccine is being distributed now, at CHC, both employees and high-risk patients will be inoculated. For CHC’s patients — many of whom come from underprivileged communities and are uninsured — it’s pivotal that CHC provide them with care as quickly as possible.
“We are already pulling data on the high-risk groups among our patient populations, and we’re watching CDC guidance on that, because now they’re starting to further tier that and show who you do first, who you do next,” Andree said. “And we’re already getting that ready so that when we do get the vaccine, we can immediately inform those patients.”
Though the first vaccines were reserved for medical personnel and those in the elderly population, medical leaders expect them to become available to more segments of the population in early 2021.
“Every increase that we have in the supply means more people sooner in the process will have access to a vaccine, so look forward to that in first quarter of (2021),” Smith said. “I’m pretty optimistic that we’re going to be able to have community vaccination probably February — could be as late as March — but we’re going to watch that very carefully.”
Just as how things had been incredibly fluid throughout 2020 regarding the virus and the response from the medical world, the same remains true in 2021, said Suzie Socier, marketing director at Paramount Urgent Care.
“Of course, the way that they’re distributing it, it could take a long time to work through everyone even though they have a protocol who is going to first receive it — it takes time,” Socier said. “And not every state is going to receive it at the same time.”
Along with patience, there also has been a call for everyone to educate themselves on the vaccine and know it is safe. There have been minor side effects that have arisen in some people — such as soreness at the injection site and temporary effects such as fatigue and headache — but experts say it’s worth it to protect yourself and others from COVID-19.
Above all, the vaccine is a way to move forward and restore something that many people have lost: Hope.
“I just look forward to the day when hope returns,” Andree said. “It provides hope for many where their hopes were changed or altered in so many ways, and it provides a new hope for many things in their lives with this possibility of return to work, return to health and return to socializing in a way that we are more familiar with prior to the pandemic.”