- February 4, 2026
-
-
Loading

Loading
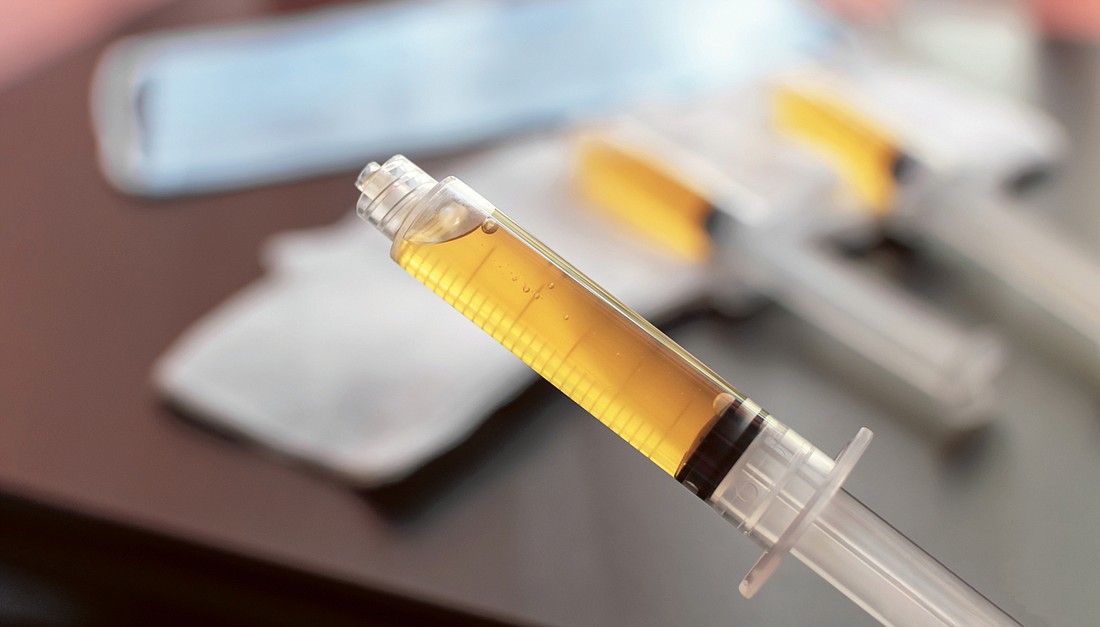
To some doctors and scientists, convalescent plasma has become known as “liquid gold,” and rightfully so.
For some COVID-19 patients who received the plasma, it has meant the difference between life and death.
In the COVID-19 era we’re living in, much attention has been focused on finding two things: effective treatments and a vaccine for the coronavirus.
When the coronavirus pandemic first hit the United States, medical professionals suddenly were faced with the challenge of how to give COVID-19 patients the best chance possible at fighting the virus. Convalescent plasma has become part of many health care professionals’ standard prescribed regimen.
Blood has four main components: plasma, red blood cells, white blood cells and platelets. Plasma is the liquid portion of the blood that helps us maintain blood pressure and volume. It also supplies critical proteins for blood clotting and immunity, as well as carries electrolytes to our muscles.
Convalescent plasma is collected from patients who have recovered from COVID-19, as those patients develop antibodies — proteins that may help fight infection — in the blood against the virus. Although not yet approved for use by the U.S. Food and Drug Administration, the agency has regulated convalescent plasma as an investigational product.
“By transfusing (survivors’) plasma into the patient still fighting the virus, it could potentially boost their immune system and help them recover,” said Susan Forbes, senior vice president of corporate communications and public relations at OneBlood. “The FDA gave blood centers emergency permission to be able to begin collecting convalescent plasma back at the end of March as an experimental procedure.”
“From a COVID standpoint, we need patients (who) have been infected with COVID, have recovered and now they have those antibodies,” said Dr. Eduardo Oliveira, executive medical director for critical care services at AdventHealth. “It is an experimental treatment in the sense that we don’t have the data yet to understand which are the patients that benefit the most.”
Oliveira said convalescent plasma has been used with some success for more than 50 years. Most recently, he said, it has been used in SARS and MERS epidemics or surges.
“We believe convalescent plasma can accelerate and help them recover much faster, and we have some patients which we believe that made a difference.” — Dr. Eduardo Oliveira, AdventHealth
“It’s not something that is just now being discovered to be helpful,” he said. “It makes sense that those antibodies produced by someone that has recovered could block viruses.”
Forbes said collection of convalescent plasma is an automated procedure that essentially removes all the components of a donor’s blood and separates it in a machine as the donation is occurring. The plasma is collected, and all other blood components are returned to the donor via a return line.
“That donation is taken to our biologics facility where we process it and prepare it to go to a hospital,” Forbes said. “At the same time, testing is going on to ensure that that donation is safe and it passes all FDA-required testing.”
The plasma is then delivered to hospitals, where it can be transfused to COVID-19 patients in need. Oliveira said medical staff observe patients to note any improvements in their breathing, blood levels and certain inflammatory markers.
“We’ve used convalescent plasma in patients (who) were not in the ICU and patients that were in the ICU,” Oliveira said. “We believe in some of those patients, it’s quite likely that the plasma has helped them not require going to the ICU or being on the mechanical ventilators — that’s what we hope. Also in patients that are very ill in the ICU on ventilators — extremely sick — we believe convalescent plasma can accelerate and help them recover much faster, and we have some patients which we believe that made a difference.”
To be eligible to donate COVID-19 convalescent plasma, Forbes said, donors must have proof that they had the virus — and have recovered — or have the antibodies. Those who had the virus and recovered must be at least 14 days symptom-free prior to donation.
At OneBlood — which services most of Florida and parts of Georgia, Alabama, South Carolina and North Carolina — donors must preregister online and provide preliminary health information. OneBlood reviews the submitted materials to ensure requirements are met, and staff reaches out to donors to schedule collection.
“We began distributing convalescent plasma in April,” Forbes said. “We were one of the very first blood centers in the country to distribute it. We will continue to distribute it for the foreseeable future because the need is ongoing, and OneBlood has experienced an over 500% increase in hospital orders for convalescent plasma.”
Forbes said a surge in COVID-19 cases combined with the fact hospitals are providing convalescent plasma earlier in treatment is driving demand to new heights.
“We’ve distributed thousands of units at this point,” Forbes said. “There’s ongoing studies about convalescent plasma with the Mayo Clinic and others. … It is something that is showing great promise in being able to help these patients. The need is now and the need is ongoing, and that’s why it’s important that people who recovered from it make it a habit and continue to donate plasma.”
“We’ve seen a shift of utilizing convalescent plasma in patients that are outside of the intensive care unit,” Oliveira said. “We are using convalescent plasma 50% more now than we were before.”

Many patients and their families believe COVID-19 convalescent plasma made a difference in their treatment regimen. Janice Moran, executive director of the American Red Cross’ Central Florida region, said her mother, who lives in a Texas nursing home, was a recipient of the plasma.
“While undergoing dialysis, the doctor felt she needed to get tested for COVID-19,” Moran said. “She tested positive. The doctor stated (although) she is not a candidate for Remdesivir, she is for convalescent plasma. The question was how quickly they would receive it, as it is in low supply around the country. Thankfully, she was able to receive the convalescent plasma in approximately 24 hours and was released from the hospital within a day or so.”
The Red Cross does not collect blood in most of Florida, Moran said, but it does partner with OneBlood on various occasions. Moran encourages local blood and plasma donors to reach out to their local blood-collection organization.
“Right now, the American Red Cross is distributing convalescent plasma products faster than donations are coming in, resulting in an emergency shortage of this potentially lifesaving treatment,” she said. “With coronavirus cases increasing across the U.S., the Red Cross has seen demand for convalescent plasma more than double over the last month. COVID-19 survivors have a unique ability to help up to three patients recover from the virus with each donation.”
Forbes said OneBlood has seen a good response from convalescent plasma donors, but what they need is a sustained response. Oliveira agreed, saying that it is a key player in helping COVID-19 patients on the road to recovery.
“Having convalescent plasma, having Remdesivir — all of that will help us support the critically ill patient that’s hospitalized,” Oliveira said. “Getting help from the community is extremely important. … We don’t want you to get infected — we want you to protect your community, as well — but if you do and you’ve recovered, you’re going to be proud of what you do today in helping our patients in the hospital.”
Dr. George Ralls, vice president of quality and clinical transformation for Orlando Health, emphasized donors are needed now more than ever.
“This is something anyone who has recovered from COVID-19 should be aware of,” Ralls said. “The goal is to have enough plasma in inventory so we don’t have to rely on a one-to-one connection. … We need to have an inventory of plasma that can be used for patients no matter where they are.”