- April 19, 2024
-
-
Loading

Loading
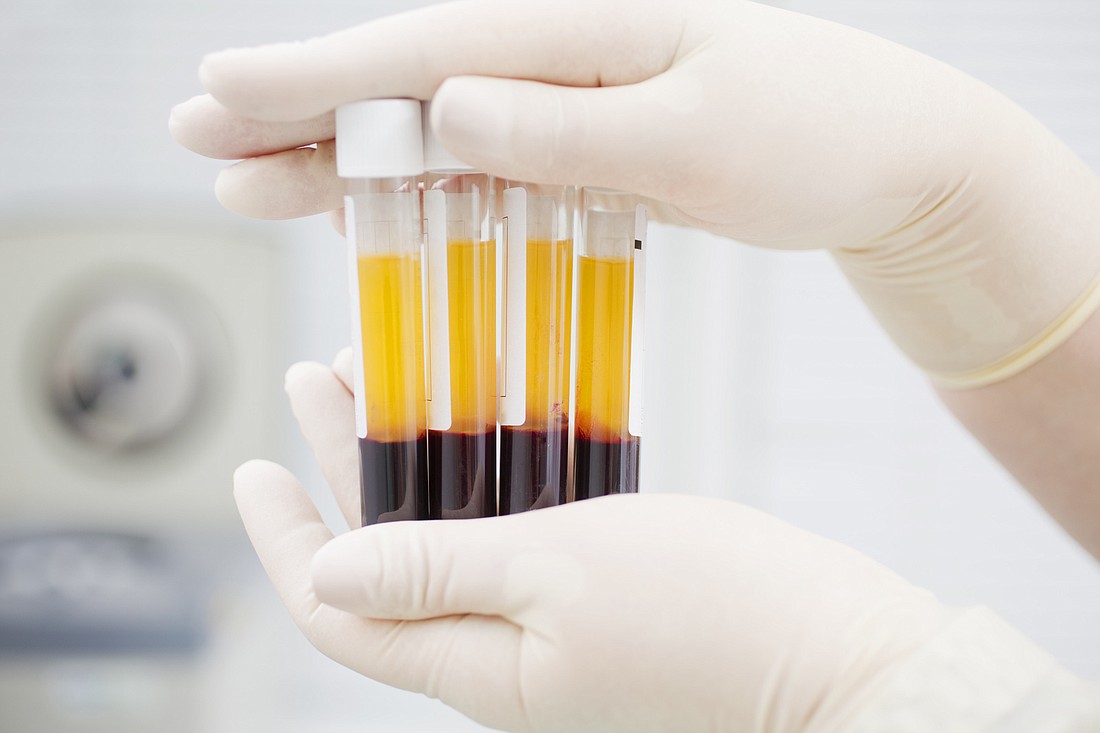
AdventHealth is offering a new treatment for COVID-19 patients, based on antibodies contained in blood plasma of patients who recently recovered from the disease.
The company also has used a 3-D printer to developed supplies to help protect health care workers.
PLASMA TREATMENT
Patients with severe cases of COVID-19 may find help in an unlikely source — the blood plasma of patients who jave recovered from the disease.
It’s called convalescent plasma, and the idea is that antibodies in the donated plasma will help fight the disease in patients who are suffering through it.
“This is an extremely exciting development that shows promise in helping our sickest patients,” said Dr. Juliana Gaitan, who is leading the project. “We’re among the first hospitals in the country to begin offering this therapy.”
The U.S. Food & Drug Administration is facilitating access to the COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening infections.
AdventHealth is working with OneBlood to solicit donations from recovered patients across the region. OneBlood will collect the plasma, which can be collected either from whole-blood or plasma-only donors.
Recovered patients who are at least 15 days out from experiencing symptoms are eligible to donate.
“We are really depending on the community for support,” Gaitan said. “As the number of COVID-19 cases increases, we expect high demand for this potentially life-saving treatment.”
Miami Mayor Francis Suarez was OneBlood’s first COVID-19 convalescent plasma donor, and less than a week later, several more people have stepped forward to donate, said officials from OneBlood.
“OneBlood is actively identifying qualified donors and arranging for their donations,” said Susan Forbes, senior vice president of corporate communications and public relations at OneBlood. “In some cases, donations have been issued to hospitals within 24 hours of a person donating.”
People who recover from coronavirus infection have developed antibodies to the virus that remain in the plasma portion of their blood. Transfusing the plasma that contains the antibodies into a person still fighting the virus can provide a boost to the patient’s immune system and potentially help them recover.
In addition to state health departments, OneBlood is working directly with their hospital partners and physicians to identify people who have recovered from the coronavirus who can be potential donors. OneBlood also has launched a social media initiative to bring heightened awareness to people who have recovered from the virus, letting them know that they are needed and could hold the potential key to helping critically ill coronavirus patients recover.
“Hospitals are eager to use this therapeutic treatment. OneBlood has the ability to help during an unprecedented time and our team is working around the clock to meet the growing demand for COVID-19 convalescent plasma,” Forbes said.
People who think they may be a candidate should visit OneBlood’s website, oneblood.org. Donors who meet the FDA criteria to be a donor will be contacted by OneBlood to coordinate their donation.
In addition to local efforts, OneBlood also is cooperating with the federal government and anticipates participating in a national-level initiative to be able to provide convalescent plasma when and where it is needed.
OneBlood is one of the largest blood centers in the country and already has the technology in place to be able to collect, test and process plasma from donors.
FACE SHIELDS
Hospital leaders, including those at AdventHealth, are scouring the world for masks and other protective equipment.
But Jodi Fails didn’t have to look far. She simply turned to a 3-D printer at AdventHealth and found an innovative solution that will help create thousands of face shields for clinicians.
Fails, a product development engineer manager at the AdventHealth Nicholson Center prototype lab in Celebration, usually uses the 3-D printer to create and test novel devices for clinicians and external companies — like models of a patient’s hip or tools to help physicians during surgery.
“It’s an honor to be able to assist our team members as they fight this pandemic,” Fails said. “We may not be providing direct patient care, but through the lab we can help protect our colleagues on the front lines.”
Fails began researching and found designs for face shields created by her fellow 3-D community online. Much attention has focused on the need for surgical masks, but face shields — clear, curved pieces of plastic attached to a headband — are also vital and in short supply. Fails soon created a successful prototype.
Fails and the Nicholson Center team then enlisted the help of academic and industry partners to mass-produce the equipment. Companies large and small are taking part, including Universal Orlando Resort, Cimquest, Taz 3D and Out of This World Embroidery.
Production of the face shields is currently at 1,000 a week, with a preliminary goal of 20,000. The shields are being distributed to AdventHealth hospitals across Central Florida. And if more production partners join the efforts, those numbers could go up and help more clinicians.